At Which Position Is Bromine Located on the Following Structure
Helpful 0 Not Helpful 0 Add. The bromine reacts with the alkene by radical chain mechanism.
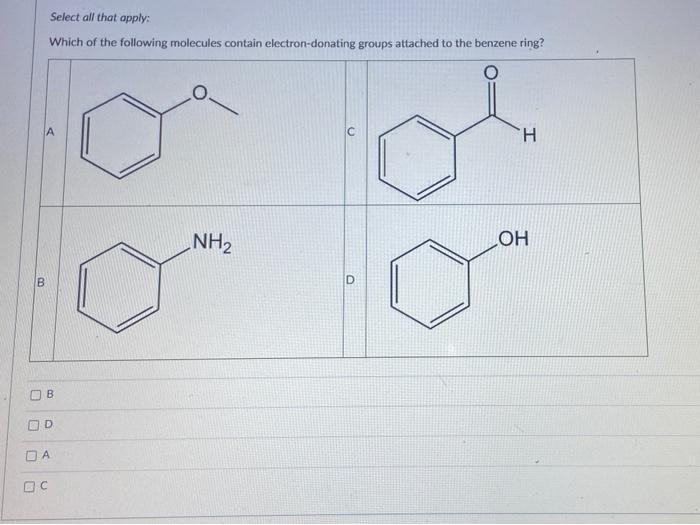
Solved If You Conducted A Bromination On The Following Chegg Com
Protons and neutrons are located in the nucleus.
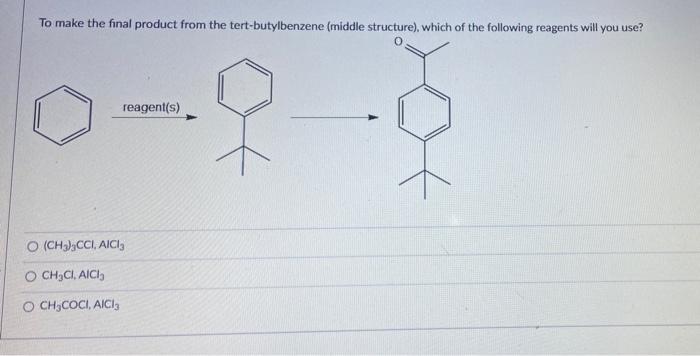
. Therefore the full name of the molecule is 4-bromopentanoic acid. Test Result Bromine Solution was orange after mixing for 10 seconds KMnO Solution was purple after 2 minutes Flammability Very little smok HNO 3 H 2 SO 4 -. CBr4 molecular geometry We can also find the electron and molecular geometry of CBr4 using the AXN method and VSEPR chart.
In the case of Bromine the abbreviated electron configuration is Ar 3d10 4s2 4p5. The atomic number is the number of protons. In the event bromination of azulene 1c produces a resonance stabilized cation 2a.
Bromine is liberated from NBS by trace amounts of HBr formed by the slow decomposition of NBS. That is the number of protons in bromine is thirty-five. Bromine Overview Bromine Complete Electron Configuration 1s2 2s2 2p6 3s2 3p6 4 s2 3 d10 4 p5 Abbreviated Electron Configuration Ar 3d10 4s2.
Construct models of alkanes haloalkanes alkenes simple amino acids and isomeric compounds. Periodic table starts at top left Atomic number 1 and ends at bottom right atomic number 118. Benzylic position H H Br HO H a Propose a mechanism to show why free-radical halogenation occurs almost exclusively at the benzylic position.
It is a volatile reddish-brown liquid that gives off suffocating vapors is corrosive to the skin and may cause severe gastroenteritis if ingested. The substituent bromine is present at fourth carbon and then prefix will be 4-bromo. The BrBr distance is 227 pm close to the gaseous BrBr distance of 228 pm and the BrBr distance between molecules is 331 pm within a layer and 399 pm between layers compare the van der Waals radius of bromine 195 pm.
Which of the following structures violates the octet rule. Bromine is the third-lightest halogen and is a fuming red-brown liquid at room temperature that evaporates readily to form a similarly coloured gas. We need to indicate the positions of the two substituents so this is expanded to cyclopentane-13.
You can use the HTML code below to embed the current 3D model in your website. Nevertheless check the complete configuration and other interesting facts about Bromine that most people dont know. Radical bromination of the following compound introduces bromine primarily at the be position next to the aro- matic ring.
When the electronegativity of one atom is lower than Feb 05 2022 Orbital-Filling Diagram for Bromine. In metals and in many other solids the atoms are arranged in regular arrays called crystals. Dibromine is a diatomic bromine.
This structure means that bromine is a very poor conductor of electricity with a conductivity of around 5 10 13 Ω 1 cm 1 just below the. If the reaction stops the monobromination Stage two stereoisomers result. Bromine is the only nonmetallic element that is liquid at ordinary temperatures.
It is a dense reddish-brown liquid which evaporates easily at room temperature to a red vapor with a strong chlorine-like odor. The forces of chemical bonding causes this repetition. Block in periodic table.
Electrons equal to protons are located in a circular shell outside the nucleus. Write structural formulas and name alkanes haloalkanes cis- and trans- isomers and optically active. The nucleus is located in the center of the atom.
The reaction is ionic and likely involves bromine rather than N-bromosuccinimide NBS 4 directly. Br Bromine is an element with position number 35 in the periodic table. Period in periodic table.
When placing the bromine in the position of the non-contaminated electron in each resonant structure giving the following products. The ground state electronic configuration of neutral bromine is Ar. Use a molecular model kit to construct these molecules and visualize their structure and three-dimensional shape.
How to Locate Bromine on Periodic Table Periodic table is arranged by atomic number number of protons in the nucleus which is same as number of electrons. The benzene ring as a branch is called a _____ group. In the IUPAC name for the following compound the -Br group located at what position of the compound is shown CH3CHBrCHCH2.
NO 2 HS O 4 H 2 O If the molecular weight of nitric acid is 6301 gmol and its density is 15129 gmL what. Bromine is a chemical element with atomic number 35 which means there are 35 protons and 35 electrons in the atomic structure. A crystal lattice is a repeating pattern of mathematical points that extends throughout space.
4p5 and the term symbol of bromine is 2P32. Step 5 of 10 c In the following structure principle functional group is carboxylic acid. Its canonical structure is 2b that is identical to 2b.
The following test results were obtained for an unknown. Bromine atoms have 35 electrons and the shell structure is 28187. Bromine is on 35th position in the Periodic Table It has 35 atomic numberIt is a halogen.
The Lewis structures in Figure 1 indicate that the ozone molecule has two equivalent resonance structures which means the electrons are delocalized. The reaction of bromine with an alkene can be detected by which of the following. The atomic number increases from left to right.
From the Lewis structure we see that the bond order for O2 is 2 a double bond whereas the bond order for. The chemical symbol for Bromine is Br. Bromine is a deep-red oily liquid with a sharp smell.
A halogen with the atomic symbol Br atomic number 35 and atomic weight 79904. The carbon atom is located in the center of the tetrahedron while the four bromine atoms are located on the vertices. The atomic number of bromine is 35.
The resonance-stabilized radical may then react Br 2 and be undergoing to halogen abstraction at any location. Bromine is less reactive than chlorine or fluorine but more reactive than iodine. A possible crystal structure of Bromine is orthorhombic structure.
Give the orbital diagram of a bromine Br atom Z 35. Bromine is used in many areas such as agricultural chemicals dyestuffs insecticides pharmaceuticals and chemical intermediates. Some uses are being phased out for environmental reasons but new uses continue to be found.
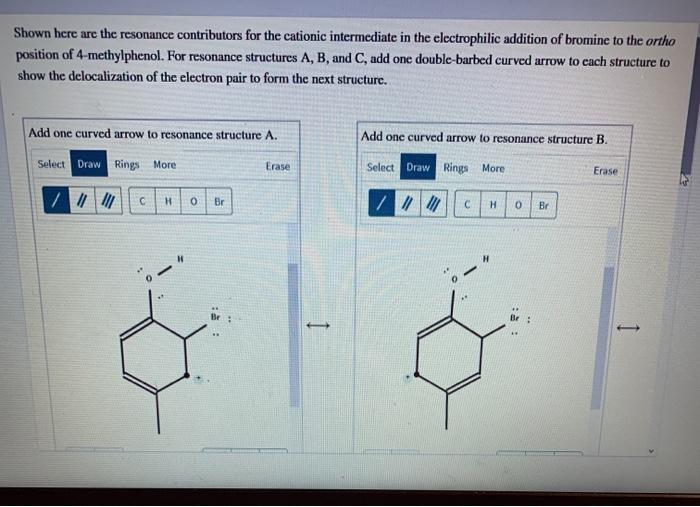
Solved Shown Here Are The Resonance Contributors For The Chegg Com
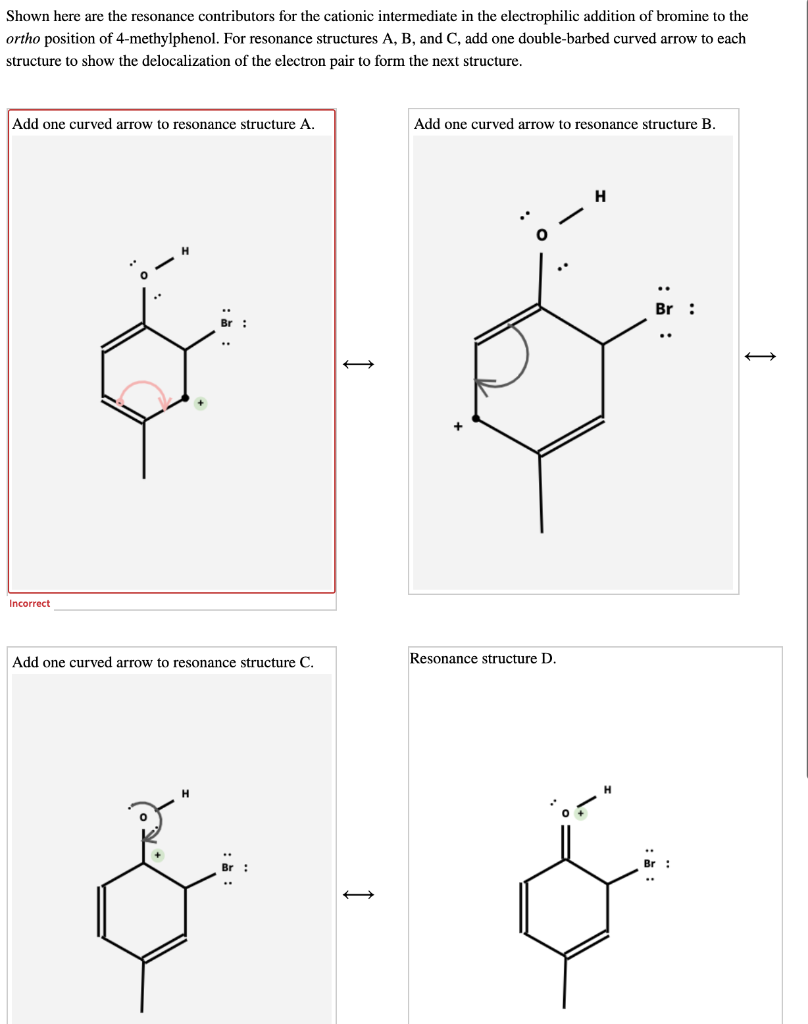
Solved Shown Here Are The Resonance Contributors For The Chegg Com
Solved If You Conducted A Bromination On The Following Chegg Com

Solved If You Conducted A Bromination On The Following Chegg Com
No comments for "At Which Position Is Bromine Located on the Following Structure"
Post a Comment